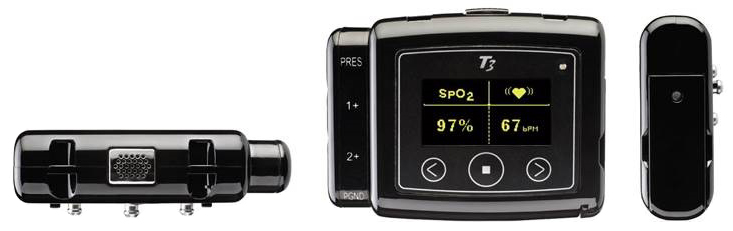
In the current situation, many sleep clinics are facing closings of their sleep operations while hospitals are moving away from in-lab sleep testing. Already an accepted technique for diagnosing Sleep Disordered Breathing (SDB), home sleep testing (HST) is emerging as a diagnostic tool throughout the US, now more than ever. During these uncertain times, HST may allow sleep clinics to clear out backlogs instead of closing the doors to patients. The Nox T3 home sleep testing device is thoughtfully designed for safe and hygienic operation offering disposable RIP belts and filtered nasal cannula, while providing high signal quality.
The Nox T3 HST
Nox Medical originally launched the Nox T3 in 2009 to help medical professionals with performing simple, but accurate home sleep tests. Since then, thousands of units have been sold globally. The Nox T3 is able to balance and incorporate a simple system that is both cost-effective and provides accurate results.
- Single patient use RIP Belts and nasal cannula with filter reducing the risk of cross-infection and possible cross contamination if handled properly.
- Calibrated RIP flow could replace the cannula if so desired1.
- Low cost of operation compared to other solutions with less capability and data
- Expandable channels and interfaces include: CPAP, EEG, ECG, EOG, PLM, and more.
- Intended for patients greater than 2 years of age.
- User friendly software with auto-scoring, manual scoring, or both.
- Integrated microphone for recording sound and snore. This is in addition to the snoring acquired through the nasal cannula.
- Easy to set up for patients with how-to videos
- Get started quickly with our self-training videos as well as web classes
The Nox T3 comes with analysis and reporting sleep software, Noxturnal, which is intended to help clinicians to diagnose various sleep disorders.
Recent research reports on the accuracy of the Noxturnal’s automatic scoring system compared to manual scoring of AHI events in Nox T3 recordings. The quote below is taken directly from the above mentioned publication, addressing the importance of such accuracy for clinical management2.
“Although current guidelines for HSAT strongly recommend manually edited scoring, the close agreement between automatic and manually edited scoring could potentially allow practitioners without sufficient resources for manual editing to rely on the automatic score for clinical management.”2
Another study published in the journal Sleep and Breathing3 concluded that the Nox T3 demonstrated very good measurement agreement compared to PSG and a high degree of sensitivity for detecting even mild OSA. In addition, recent publication report on the usage of the Nox T3 in a pediatric population4 to speed the route to sleep-disorder therapy. Another publication reports on the usage of the Nox T3 in a COPD population for diagnosis of OSA5.
The sophisticated home sleep monitoring device, the Nox T3 by Nox Medical, can detect subtle changes in breathing parameters throughout the night. The downloadable data gives clinicians a comprehensive picture of the abdominal and thoracic respiratory effort and derived flow, nasal cannula flow, body position and activity, as well as blood oxygen levels, and pulse. With these details, practitioners have the ability to refine their diagnosis.
Contact Us
Please contact us to learn more about the Nox T3 sleep diagnostic solution.
References
- Berry RB, Quan SF, Abreu AR, et al; for the American Academy of Sleep Medicine. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. Version 2.6. Darien, IL: American Academy of Sleep Medicine; 2020.
- Xu, L., Han, F., Keenan, B. T., Kneeland-Szanto, E., Yan, H., Dong, X., … Kuna, S. T. (2017). Validation of the Nox-T3 portable monitor for diagnosis of obstructive sleep apnea in Chinese adults. Journal of Clinical Sleep Medicine, 13(5), 675–683. https://doi.org/10.5664/jcsm.6582
- Cairns, A., Wickwire, E., Schaefer, E., & Nyanjom, D. (2014). A pilot validation study for the NOX T3TM portable monitor for the detection of OSA. Sleep and Breathing, 18(3), 609–614. https://doi.org/10.1007/s11325-013-0924-2
- Singh, G., Hardin, K., Bang, H., & Nandalike, K. (2019). The feasibility and utility of level III portable sleep studies in the pediatric inpatient setting. Journal of Clinical Sleep Medicine, 15(7), 985–990. https://doi.org/10.5664/jcsm.7878
- Chang, Y., Xu, L., Han, F., Keenan, B. T., Kneeland-Szanto, E., Zhang, R., … Kuna, S. T. (2019). Validation of the NOX-T3 portable monitor for diagnosis of obstructive sleep apnea in patients with chronic obstructive pulmonary disease. Journal of Clinical Sleep Medicine, 15(4), 587–596. https://doi.org/10.5664/jcsm.7720
Topic: Industry News