More than 300 scientific articles have been published in the past decade using Nox Medical’s sleep testing solutions.
These articles form a substantial scientific evidence base for our products, and have been meticulously collated by the product research team as part of the process to register Nox Medical’s products under the new EU medical device regulations. The literature on Nox’s products is summarized below.
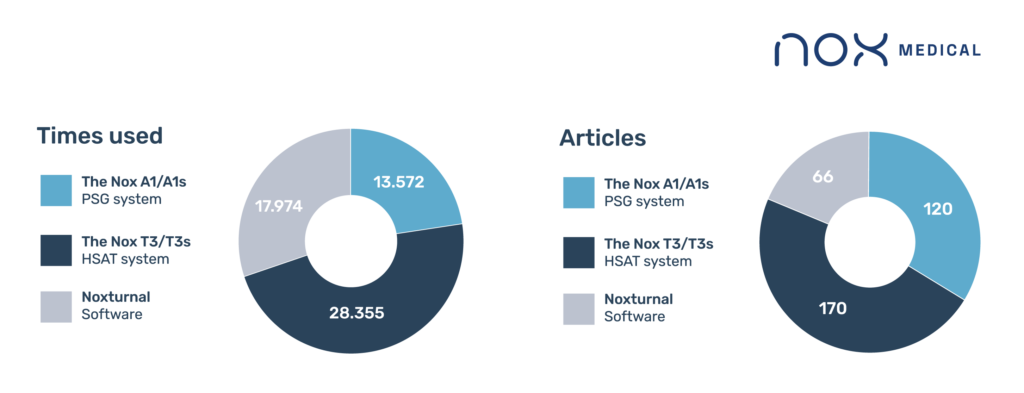
Nox Medical’s polysomnography (PSG) system, the A1/A1s, has been used an estimated 13,572 times in 120 scientific articles. These articles used data from healthy children and adults, to elite athletes, patients with obstructive sleep apnea and sleep bruxism to those with neurological, psychiatric, or cardiorespiratory conditions.
Approximately two-thirds of the patients included in the scientific literature we reviewed were male and their ages varied from 2 to 90 years old. Overall, the Nox A1/A1s was described as a reliable recording device for both at-home and in-lab studies with a low failure rate and high-quality signals.
The Nox T3/T3s HSAT system has been used an estimated 28,355 times reported in more than 170 scientific articles. Much like the Nox A1/A1s, the articles provide data from a wide variety of patient populations also including roughly two-thirds male participants and both children and adults.
According to the literature, the Nox T3/T3s has primarily been used to screen for sleep-disordered breathing, but has also been used to validate other new screening methods and develop deep learning algorithms to improve the accuracy of home sleep testing.
Across the reviewed articles it was clear that the Nox T3/T3s provides high-quality information and has a low failure rate. Some articles even reported that the device is easy to use and comfortable to wear, with the potential to enhance efficiency of sleep diagnostic pathways.
Finally, Noxturnal software was specifically discussed in 66 articles reporting on an estimated 17,974 uses. In the articles, the Noxturnal software was used in combination with either the Nox A1/A1s or the Nox T3/T3s to configure the devices, download the data from them and either run automatic analyses or provide an interface for manual scoring.
The most reported feature used in Noxturnal was the automatic respiratory flow analysis, which estimates clinical parameters like the apnea-hypopnea index and oxygen desaturation index that can then be manually reviewed and corrected.
Nox’s BodySleep analysis,* which extracts estimated sleep stages from Nox T3/T3s sleep studies without needing EEG signals, was also reported in several articles (note: BodySleep is not available in the US). At least 13 scientific articles have directly compared automatic analyses from the Noxturnal software to manual scoring, showing good to excellent accuracy.This extensive scientific literature cements that Nox Medical’s products are state-of-the-art and are widely considered the “gold standard” reference test in sleep research. The Nox A1/A1s, Nox T3/T3s and Noxturnal software have been used thousands of times with minimal failures and are setting new standards for both at-home and in-lab sleep diagnostics.
*Nox BodySleep is currently not available in the United States
Topic: Industry News