The Nox T3s home sleep testing system features Nox Medical’s newest innovation, the Nox BodySleep analysis*, which allows physicians to assess patients’ sleep time by distinguishing REM, NREM and Wake in a home environment without complicating or compromising the patient experience. The Nox BodySleep does not require traditional EEG, EOG, and EMG signals typically used in a PSG study to determine changes during sleep stages.
The Nox BodySleep analysis, available within the Noxturnal software, is a new and validated method that uses an artificial intelligence (AI) algorithm to analyze respiratory and actigraphy data for more accurate Apnea-Hypopnea Index (AHI) estimation. The method is intended to classify 30-second epochs into the states of REM sleep, NREM sleep and Wake. It uses respiratory inductance plethysmography (RIP) signals derived from the Nox RIP belts and actigraphy to measure the impact of brain state change on the body and estimates sleep states from those signals. The clinical purpose of Nox BodySleep is to get a more accurate estimate of sleep time during a home sleep study by correctly classifying wake in a home sleep test.
The Physiological Principles Behind Nox BodySleep
The Nox BodySleep analysis uses robust physiological parameters, which are unlikely to fail during a sleep study (Montazeri et al 2020, Ioan et al 2020). The primary input to the analysis is respiratory data processed through advanced algorithms utilizing Nox calibrated RIP technology. The Nox BodySleep uses the abdominal and thoracic RIP signals, respiratory rate, which is derived from the RIP signals, and actigraphy as input signals. Those physiological parameters are easily obtained in a home sleep study with high patient comfort while showing good clinical accuracy in determining the AHI and SDB disease severity. Furthermore, the Nox BodySleep uses these signals to assess sleep time.
Proof-of-Principle Study
In a proof-of-principle study recently published, the novel Nox BodySleep algorithm was tested for sensitivity and specificity along with its performance on distinguishing between REM, NREM and wakefulness. The article concludes that the Nox BodySleep algorithm could be an appropriate tool to increase the diagnostic accuracy of portable monitoring. Validation of the Nox BodySleep is compared to manually scored PSG.
In the study, when using the Nox BodySleep analysis to determine AHI there was an excellent correlation with manually scored AHI (r=0.91). The algorithm was also able to help classify patients into AHI groups depending on SDB severity with good agreement with manual scoring. According to the study, the Nox BodySleep shows good estimation of total sleep time as the algorithm estimation correlated well with manual scoring of sleep time (Pearson correlation of r=0.81). Furthermore, the authors concluded that the Nox BodySleep algorithm showed a good clinical accuracy in estimating the first occurrence of sleep during waking-sleep transition.
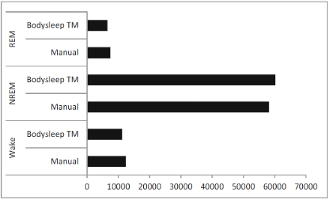
The figure below shows the comparison between the number of epochs scored with the Nox BodySleep algorithm and epochs scored manually from PSG data. Moreover, a good agreement between the Nox BodySleep and manual scoring was demonstrated from an epoch-by-epoch comparison (Cohen’s kappa = 0.62).
Taken all together, the Nox BodySleep algorithm uses physiological parameters that are easily obtained with high patient comfort and show good clinical accuracy in estimating sleep states, total sleep time for determining the AHI and SDB disease severity. The Nox BodySleep algorithm was tested for BMI, sex and AHI ranges (0-5 – 5-15 – over 15). No significant changes in performance of the algorithm were found between these groups.
The Nox BodySleep analysis is available in the Noxturnal Software and compatible with the Nox T3s home sleep testing system by Nox Medical.
*Nox BodySleep is not available in the United States.
Topic: Research & Publications