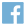In the evolving field of sleep medicine, choosing the right diagnostic system for your sleep lab is essential. Whether you are opening a new sleep lab or switching from an outdated polysomnography (PSG) system, the diagnostic technology that you choose for your sleep lab is one of the most important decisions you will make. The Nox A1s PSG System could be the solution you’re looking for, offering patient-centered design and versatile functionality for both in-lab and home settings. This article explores whether the Nox A1s is the right fit for your needs, highlighting its streamlined setup, versatile functionality, and advanced technical capabilities.
The Nox A1s is an advanced approach to polysomnography (PSG) that simplifies in-lab sleep studies while seamlessly extending into home environments. With its lightweight, tether-free design, the system allows sleep professionals to conduct PSG studies that adhere to the AASM standards for lab-based sleep studies1. It records the same high-quality electroencephalogram (EEG) signals as a traditional headbox but without the hassle, integrating all necessary EEG channels into a single, streamlined head cable.
Reported in over 13,500 uses across 120 scientific articles, the Nox A1s has been tested with diverse populations, ranging from healthy individuals to patients with obstructive sleep apnea, sleep bruxism, and various neurological, psychiatric, or cardiorespiratory conditions.
In the following section, we’ll explore the key features, capabilities, and technical specifications of the Nox A1s PSG system, helping you determine if it’s the right choice for your sleep lab.
Flexibility for Patient Comfort
The Nox A1s has an untethered design, replacing the traditional headbox and cumbersome cables with a single integrated connection. Wires are still present, but they are reduced, and attached only to the body. Bluetooth transmits all sleep study data while a backup copy is also stored on an internal hard drive for additional peace of mind. These wireless capabilities speed up the setup process and allow patients to undergo sleep testing without being attached to the bedside. As a result, patients experience freedom of movement during sleep studies.
Efficiency Redefined
From its streamlined installation process to its user-friendly operation, the Nox A1s is optimized for efficiency at every step. Sleep technologists can use the Noxturnal App on a tablet that syncs with the Nox A1s PSG system in real time to perform bio-calibration, impedance checks, and live trace viewing while staying in direct communication with the patient. Meanwhile, the Noxturnal desktop software provides a modern and intuitive interface for scoring, automatic analysis, and advanced reporting, with easy editing capabilities. Clinicians can effortlessly conduct customized MSLT and MWT sleep studies, as well as split night and titration studies. Moreover, Nox provides around-the-clock access to technical support’s expert technicians, ensuring that support is always available when needed.

Cost Savings with Integrated Components
The Nox A1s integrates several components typically purchased separately, which may contribute to cost savings and streamlined operations over the device’s lifetime. The only single-patient components that must be replaced are the nasal cannula and RIP sensors.
Nox A1s integrated technology:
- RIP system
- PTAF
- Snore mic
- Body Position
- Audo recording device
Nox RIP Technology
The Nox A1s PSG System is equipped with a range of advanced features designed to deliver high-quality sleep diagnostics. The Nox A1s uses two single-patient RIP belts which record changes in the volume of the abdomen and chest. The Nox RIP is a patented, automatic, and continuously calibrated technology that eliminates the need for labor-intensive and intrusive manual calibrations to address position changes during the study. Studies show that the Nox RIP can be a reliable backup signal for cannula flow2,3,4. For a detailed breakdown of all technical specifications, click here.
Integration with Lifelines R40 Amplifier
Nox A1s PSG system integrates with Lifelines R40 amplifier enabling 10-20 referential and bipolar EEG recording for enhanced data collection, along with the inclusion of 2 additional bipolar channels.
Designed for the Lab, Ready for Home Use
While the Nox A1s is designed for in-laboratory polysomnography sleep testing, it is also equipped for Type II unattended, ambulatory polysomnography sleep studies, enabling its use in various settings, including different hospital wings or a patient’s home. Research, published in Sleep and Biological Rhythms, found a similar sensor quality for airflow, oxygen, and respiratory effort for home and in-lab studies. Additionally, the apnea–hypopnea index scores did not differ. This forward-thinking design ensures that the Nox A1s is fully equipped to meet the evolving demands of sleep diagnostics.

Trusted Partner in Sleep Diagnostics
For over 15 years, Nox has been a leader in sleep diagnostic technology, and with the Nox A1s, we continue to shape the future of sleep medicine. Institutions like Valley Sleep Center, Arizona and Tampa General Hospital, Florida are among the many across the United States that rely on the Nox A1s for in-lab PSG sleep testing. Modern sleep centers seeking to upgrade their polysomnography systems with cutting-edge technology may choose the Nox A1s as a dependable solution.
Discover how the Nox A1s PSG system matches up with your sleep lab’s needs, a system that is designed for patient comfort and optimized workflow efficiency with its advanced hardware and software features. Contact our team to schedule a demo of the Nox A1s PSG system.
- Troester MM, Quan SF, Berry RB, et al. For the American Academy of Sleep Medicine. The AASM Manual for the Scoring of Sleep and Associated Events; Rules, Terminology and Technical Specifications. Version 3. Darien, IL: American Academy of Sleep Medicine; 2023.
- Chang Y, Xu L, Han F, Keenan BT, Kneeland-Szanto E, Zhang R, Zhang W, Yu Y, Zuo Y, Pack AI, Kuna ST. Validation of the Nox-T3 portable monitor for diagnosis of obstructive sleep apnea in patients with chronic obstructive pulmonary disease. J Clin Sleep Med. 2019;15(4):587–596. https://doi.org/10.5664/jcsm.7720
- Magalang UJ, Johns JN, Wood KA, Mindel JW, Lim DC, Bittencourt LR, Chen NH, Cistulli PA, Gíslason T, Arnardottir ES, Penzel T, Tufik S, Pack AI. Home sleep apnea testing: comparison of manual and automated scoring across international sleep centers. Sleep Breath. 2019 Mar;23(1):25-31. doi: 10.1007/s11325-018-1715-6.
- Xu L, Han F, Keenan BT, Kneeland-Szanto E, Yan H, Dong X, Chang Y, Zhao L, Zhang X, Li J, Pack AI, Kuna ST. Validation of the Nox-T3 Portable Monitor for Diagnosis of Obstructive Sleep Apnea in Chinese Adults. J Clin Sleep Med. 2017 May 15;13(5):675-683. doi: 10.5664/jcsm.6582.
Topic: Nox A1 PSG System