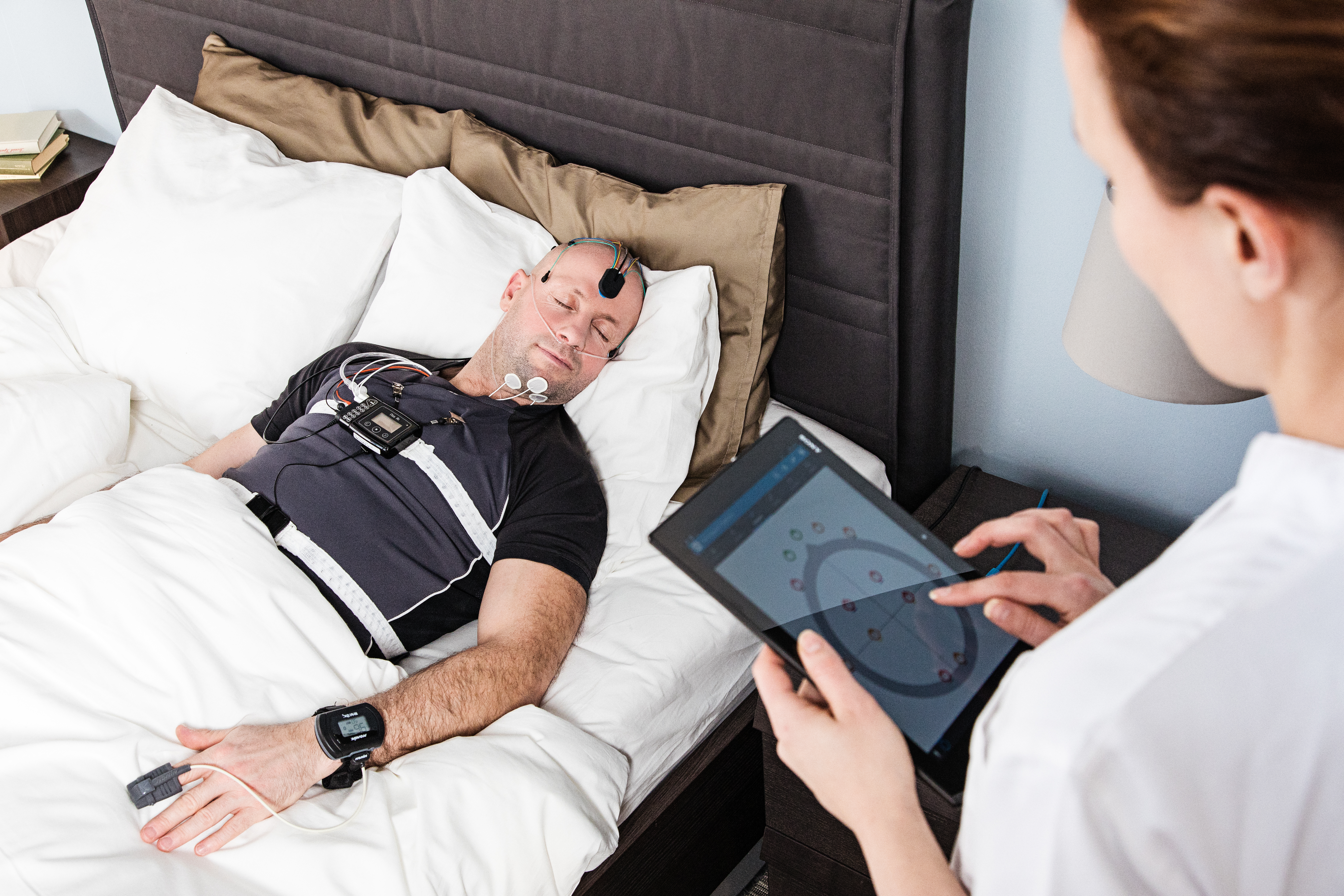
Nox Medical has officially launched its innovative polysomnographic system, the Nox A1, in the U.S. and Canada. The Nox A1 is a powerful, ambulatory PSG device that combines thoughtful design with clinical accuracy, allowing sleep labs to maximize their investment with multiple functions in one single device.
Like all Nox Medical devices, the Nox A1 was created with patient ease and comfort at the forefront. The system’s wireless design allows patients to move freely while undergoing testing and even enables the device to be used for at-home testing as needed. In addition to its dual in-lab and at-home functionality, the Nox A1 can perform Level I, Level II and Level III sleep studies, making it one of the most versatile devices on the market.
“We take great pride in not only the comfort, but the efficiency of our devices,” said Pétur Már Halldórsson, CEO of Nox Medical. “
Unlike traditional PSG systems, the Nox A1’s wireless hookup design eliminates the discomfort and inconvenience of being tethered to the bed. Plus, with minimal cables and straps, it is suitable for diverse patient population.
“The Nox A1’s benefits extend to sleep technicians as well, who can easily review the sleep study data in real time using the Noxturnal app and software, which provide continuous impedance control. The device’s fluency, from hookup to software, helps to increase efficiency and even provides sleep tech the capacity to attend to multiple patients simultaneously.”
Beyond its ease of use, the Nox A1 and its accompanying Noxturnal software utilize advanced technology that provides automatic analyses of sleep study data. In published papers1 2, the Nox A1 System has demonstrated a strong correlation between its built-in algorithm for respiratory scoring and manually scored Apnea-Hypopnea Indexes (AHI). The Noxturnal software also includes an automatic sleep staging algorithm intended to estimate total sleep time. Plus, the device itself is outfitted with two RIP (respiratory inductance plethysmography) belts, which provide an alternative for scoring apneas and hypopneas, as well as a useful backup signal if the primary nasal cannula signal fails or is unreliable.
“At Nox Health, clinical accuracy is paramount in everything we do,” Halldórsson said. “Our goal is to allow sleep providers to better assess, diagnose and treat the entire range of sleep health issues while also eliminating common pain points, including the need for re-testing. The Nox A1 simplifies the workflow of sleep studies while also delivering highly secure and precise measurements.
The Nox A1 first launched in Europe in 2014 and has since earned a reputation as a versatile, cost-effective testing and diagnostic solution that delivers a positive experience for both patients and providers. Through innovative thinking and collaboration with industry-leading physicians, Nox Medical has reimagined polysomnography in terms of ergonomics, scalability and technology.
The Nox A1 PSG System is now available in North America. For more information, visit noxmedical.com/products/nox-a1-psg-system.
1: Magalang, U. J., Johns, J. N., Wood, K. A., Mindel, J. W., Lim, D. C., Bittencourt, L. R., … Pack, A. I. (2018). Home sleep apnea testing: comparison of manual and automated scoring across international sleep centers.
2: Xu, L., Han, F., Keenan, B. T., Kneeland-Szanto, E., Yan, H., Dong, X., … Kuna, S. T. (2017). Validation of the Nox-T3 portable monitor for diagnosis of obstructive sleep apnea in Chinese adults. Journal of Clinical Sleep Medicine, 13(5), 675–683. https://doi.org/10.5664/jcsm.6582
Topic: Company Updates